PEPPSI
PEPPSI is an abbreviation for pyridine-enhanced precatalyst preparation stabilization and initiation. It refers to a family of commercially available[1][2][3] palladium catalysts developed around 2005 by Prof. Michael G. Organ and co-workers at York University,[4][5] which can accelerate various carbon-carbon and carbon-heteroatom[6] bond forming cross-coupling reactions. In comparison to many alternative palladium catalysts, Pd-PEPPSI-type complexes are stable to air and moisture and are relatively easy to synthesize and handle.
Structure and synthesis
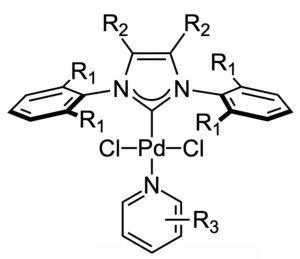
In the basic structure of Pd-PEPPSI, R1 can be a methyl (CH3, Me), ethyl (C2H5, Et), isopropyl (C3H7, iPr), isopentyl (C5H11, iPent), or isoheptyl (C7H15, iHept) group, and the resulting catalysts are thus labeled as PEPPSI-IMes, PEPPSI-IEt, PEPPSI-IPr, PEPPSI-IPent, and PEPPSI-IHept respectively, with or without "Pd-" added in front.[7] Commonly used PEPPSI catalysts such as Pd-PEPPSI-IPr[8] contain an unsubstituted imidazole core (R2=H) and a 3-chloro substituted pyridine ligand (R3=3-Cl). However, structural modifications of the imidazole backbone[9][10][11][12][13] and pyridine ligand[7][10][11] can profoundly affect the catalytic activity of these complexes.
The synthesis and structure of Pd-PEPPSI catalysts were presented in 2005[4][1] and published in 2006.[14] PEPPSI catalysts are organopalladium complexes containing N-heterocyclic carbene (NHC) ligands. They can be obtained by reacting an imidazolium salt, palladium(II) chloride, and potassium carbonate in 3-chloropyridine as a solvent, under vigorous stirring at 80 °C for 16 hours in air. The yield of PEPPSI in this reaction is 97–98%.[1][14] Contrary to other common palladium-based catalysts, such as tetrakis(triphenylphosphine)palladium(0), PEPPSI is stable to exposure to air[15] and moisture.[16] Even heating in dimethyl sulfoxide at 120 °C for hours does not result in significant decomposition or deactivation of PEPPSI catalysts.[1]
Properties and applications
PEPPSI can catalyze various palladium cross-coupling reactions including Negishi coupling,[15] Suzuki coupling, Sonogashira coupling, Kumada coupling,[17] and the Buchwald–Hartwig amination[6] as well as aryl sulfination[18][10][6] and the Heck reaction.[1][19] In Negishi coupling, PEPPSI promotes reaction of alkyl halides, aryl halides or alkyl sulfonates with alkylzinc halides,[20] and the important advantage of PEPPSI over alternative catalysts is that the reaction can be carried out in a general chemical laboratory, without a glove box. PEPPSI contains palladium in the +2 oxidation state and is thus a "precatalyst", that is the metal must be reduced to the active Pd(0) form in order to enter the cross-coupling catalytic cycle. This is usually achieved in situ in the presence of active transmetalating agents such as organo-magnesium, -zinc, -tin, or -boron reagents.[7] Once activated, the NHC-Pd(0) species becomes rather air-sensitive.[15][1][21][22]
References
- ^ a b c d e f PEPPSI Catalysts, Sigma-Aldrich
- ^ PEPPSI™-IPent for Demanding Cross-Coupling Reactions, Sigma-Aldrich
- ^ PEPPSI Catalyst, Sigma-Aldrich ChemFiles
- ^ a b Hadei, N.; Kantchev, E.A.B.; O'Brien, C.J.; Chass, G.; Hunter, H.H.; Penner, G.; Hopkinson, A.C.; Organ, M.G. (2005). Rational catalyst design and its application in sp3-sp3 couplings. 230th ACS National Meeting. Washington, DC: American Chemical Society. pp. Abstract 308.
- ^ Hadei, N.; Kantchev, E.A.B.; O'Brien, C.J.; Organ, M.G. (2005). "The First Negishi Cross-Coupling Reaction of Two Alkyl Centers Utilizing a Pd−N-Heterocyclic Carbene (NHC) Catalyst". Org. Lett. 7: 3805–3807. doi:10.1021/ol0514909. PMID 16092880.
- ^ a b c Valente, C.; Pompeo, M.; Sayah, M.; Organ, M.G. (2014). "Carbon–Heteroatom Coupling Using Pd-PEPPSI Complexes". Organic Process Research & Development. 18: 180–190. doi:10.1021/op400278d.
- ^ a b c Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E.A.B.; O'Brien, C.J.; Lough, A.; Organ, M.G. (2010). "Structure-Activity Relationship Analysis of Pd-PEPPSI Complexes in Cross-Couplings: A Close Inspection of the Catalytic Cycle and the Precatalyst Activation Model". Chem. Eur. J. 16: 10844–10853. doi:10.1002/chem.201000138. PMID 20665575.
- ^ PEPPSI™-IPr catalyst, Sigma-Aldrich
- ^ Pompeo, M.; Froese, R.D.J; Hadei, N.; Organ, M.G. (2012). "Pd-PEPPSI-IPentCl: A Highly Effective Catalyst for the Selective Cross-Coupling of Secondary Organozinc Reagents". Angew. Chem. Int. Ed. 51: 11354–11357. doi:10.1002/anie.201205747. PMID 23038603.
- ^ a b c Sayah, M.; Lough, A.J.; Organ, M.G. (2013). "Sulfination by Using Pd-PEPPSI Complexes: Studies into Precatalyst Activation, Cationic and Solvent Effects and the Role of Butoxide Base". Chem. Eur. J. 19: 2749–2756. doi:10.1002/chem.201203142. PMID 23296748.
- ^ a b Pompeo, M.; Farmer, J.L.; Froese, R.D.J; Organ, M.G. (2014). "Room-Temperature Amination of Deactivated Aniline and Aryl Halide Partners with Carbonate Base Using a Pd-PEPPSI-IPentCl-o-Picoline Catalyst". Angew. Chem. Int. Ed. 53: 3223–3226. doi:10.1002/anie.201310457. PMID 24677620.
- ^ Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M.J.; Pompeo, M.; Froese, R.D.J.; Organ, M.G. (2015). "The Selective Cross-Coupling of Secondary Alkyl Zinc Reagents to Five-Membered-Ring Heterocycles Using Pd-PEPPSI-IHeptCl". Angew. Chem. Int. Ed. 127: 9638–9642. doi:10.1002/anie.201503941. PMID 26110577.
- ^ Lu, D.-D.; Xu, X.-X.; Liu, F.-S. (2017). "Bulky Yet Flexible Pd-PEPPSI-IPentAn for the Synthesis of Sterically Hindered Biaryls in Air". J. Org. Chem. 82: 10898–10911. doi:10.1021/acs.joc.7b01711. PMID 28925697.
- ^ a b O'Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. (2006). "Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction". Chem. Eur. J. 12: 4743–8. doi:10.1002/chem.200600251. PMID 16568494.
- ^ a b c Li, Jia Jack; Corey, E.J. (2009). Name reactions for homologations, Part 1. John Wiley and Sons. ISBN 978-0-470-48701-3.
- ^ Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.G. (2010). "Pd-PEPPSI Complexes and the Negishi Reaction". Eur. J. Org. Chem.: 4343–4354. doi:10.1002/ejoc.201000359.
- ^ Ackerman, Lutz (2009). Modern Arylation Methods. Verlag: Wiley-VCH. doi:10.1002/9783527627325. ISBN 9783527319374.
- ^ Sayah, M.; Organ, M.G. (2011). "Carbon–Sulfur Bond Formation of Challenging Substrates at Low Temperature by Using Pd-PEPPSI-IPent". Chem. Eur. J. 12: 11719–11722. doi:10.1002/chem.201102158. PMID 21898625.
- ^ Luis, Santiago V.; Garcia-Verdugo, Eduardo (2009). Chemical Reactions and Processes Under Flow Conditions. Green Chemistry Series. Royal Society of Chemistry. doi:10.1039/9781847559739. ISBN 978-0-85404-192-3.
- ^ Cazin, Catherine S.J. (2010). N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis. Catalysis by Metal Complexes. Vol. 32. Netherlands: Springer. pp. 169–173. doi:10.1007/978-90-481-2866-2. ISBN 978-90-481-2866-2.
- ^ Organ, M.G.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E.A.; O'Brien, C.J.; Valente, C. (2006). "A User-Friendly, All-Purpose Pd–NHC (NHC=N-Heterocyclic Carbene) Precatalyst for the Negishi Reaction: A Step Towards a Universal Cross-Coupling Catalyst". Chem. Eur. J. 12: 4749–4755. doi:10.1002/chem.200600206. PMID 16568493.
- ^ PEPPSI: Instructions for Use, Sigma-Aldrich