Dodecamethylcyclohexasilane
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
| IUPAC name
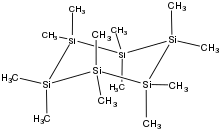
1,1,2,2,3,3,4,4,5,5,6,6-dodecamethylhexasilinane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Si6(CH3)12 | |
| Molar mass | 348.930 g·mol−1 |
| Appearance | colorless solid |
| Density | 0.988 g/cm3 |
| Melting point | 254–257 °C (489–495 °F; 527–530 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dodecamethylcyclohexasilane is the organosilicon compound with the formula Si6(CH3)12. It is one of the more readily prepared and easily handled polysilanes. Dodecamethylcyclohexasilane is produced by reduction of dimethyldichlorosilane with sodium-potassium alloy:[1]
- 6 (CH3)2SiCl2 + 12 M → (CH3)12Si6 + 12 MCl
where M is Na or K. The reaction also produces polydimethylsilane and decamethylpentasilane.[clarification needed]
The chair conformer was confirmed by X-ray crystallography.[2]
Reactions
Dodecamethylcyclohexasilane reacts with potassium tert-butoxide to give the potassium derivative:[3]
- (CH3)12Si6 + KOC(CH3)3 → K(CH3)11Si6 + CH3OC(CH3)3
References
- ^ West, Robert; Brough, Lawrence; Wojnowski, Wieslaw (1979). "Dodecamethylcyclohexasilane". Inorganic Syntheses. 19: 265–268. doi:10.1002/9780470132500.ch62. ISBN 9780470132500.
- ^ Omatsu, Yamato; Mizuhata, Yoshiyuki; Tokitoh, Norihiro (2018). "Synthesis of Dodecaallylhexasilacyclohexane and Its Convertibility". Zeitschrift für Anorganische und Allgemeine Chemie. 644 (17): 930–934. doi:10.1002/zaac.201800171. S2CID 105286121.
- ^ Palitzsch, Wolfram; Beyer, Christian; Böhme, Uwe; Rittmeister, Ben; Roewer, Gerhard (1999). "Preparation, Characterization, and Properties of Various Novel Ionic Derivatives of Pentacarbonyltungsten". European Journal of Inorganic Chemistry. 1999 (10): 1813–1820. doi:10.1002/(SICI)1099-0682(199910)1999:10<1813::AID-EJIC1813>3.0.CO;2-D.