Dangote Refinery
The Dangote Refinery is an oil refinery owned by Aliko Dangote that is under construction in Lekki, Nigeria. When completed, it is expected to have the capacity to process about 650,000 barrels per day of crude oil, making it the largest single-train refinery in the world. The investment is over 25 billion US dollars.[1]
History
Nigerian businessman Aliko Dangote unveiled early plans for the refinery in September 2013, when he announced that he had secured about $3.3 billion in financing for the project.[2] At the time, the refinery was estimated to cost about $9 billion, of which $3 billion would be invested by the Dangote Group and the remainder via commercial loans, and begin production in 2016.[2] However, after a change in location to Lekki, construction of the refinery did not begin until 2016 with excavation and infrastructure preparation, and the planned completion was pushed back to late 2018.[3][1]
In July 2017, major structural construction began, and Dangote estimated that the refinery would be mechanically complete in late 2019 and commissioned in early 2020.[1] According to Reuters, citing sources familiar with the project, construction was likely to take at least twice as long as Dangote publicly stated, with partial refining capability not likely to be achieved until 2022.[1] An associated project at the site of the refinery, a urea fertilizer factory, was scheduled to begin operation in late 2018 and produce about three million tons of urea annually.[4] In 2018 the project was expected to cost up to $15 billion in total, with $10 billion invested in the refinery, $2.5 billion in the fertilizer factory, and $2.5 billion in pipeline infrastructure.[4]
In July 2022, Dangote - Nigeria's richest resident - had to borrow 187 billion naira (about 442 million USD) at 12.75% resp. 13.5% p.a. to complete the refinery.[5] Fitch Ratings noted that the refinery's start date has been postponed three times in four years and feared diminished investor confidence if operations do not begin in 2023.[5] At the same time, all of the four refineries of the state-owned oil company NNPC (in Kaduna, Port Harcourt[6] and Warri) are idle and expect to process crude oil again in 2023 after "revamping".[7]
Facility
The refinery is situated on a 6,180 acres (2,500 hectares) site at the Lekki Free Trade Zone, Lekki, Lagos State. It is supplied with crude oil by the largest sub-sea pipeline infrastructure of the world (1,100 km long). When fully operational it will provide 135,000 permanent jobs in the region.[citation needed]
High complexity
The Dangote Oil Refinery will have a Nelson complexity index of 10.5 which means that it will be more complex than most refineries in the United States (average 9.5) or Europe (average 6.5).[8] (The largest refinery of the world, the Jamnagar Refinery in India, has a complexity of 21.1.) The Nelson complexity index basically increases with the number and capacity of chemical procedures after the distillation, e.g. hydrocracking, NHT, CCR, RFCC, polymerization etc.
Among others, the refinery will run these refinery processes (please find an illustration of the chemical processes in the gallery below):[9][10][11]
| Procedure | Lines | Licensor | |
|---|---|---|---|
| Crude Distillation Unit (CDU)[12]
Input: crude oil, Output: combustible gases, naphtha (light/heavy), jet fuel, Diesel fuel, heavy oil, residue fluid Purpose: separating crude oil components by molecule length / boiling temperature |
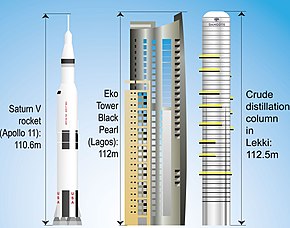
It is the first processing step in nearly all petroleum refineries (see image below). The CDU separates the different components of crude oil by their boiling points. - In the desalter salt is being removed from the crude oil. In a single preheat train the crude oil is warmed up[13] by using heat from different procedures on this list or burning fuel from own making, like LPG.[14] After this the crude oil is heated up and led into the distilling column. There lighter crude oil components (gases like methane, ethane, propane and butane)[15] and naphthas (light naphtha like pentane, hexane and heptane and heavy naphtha like octane /C8 up to C12) travel up the column, heavier oils (C25 and higher) and residue leave at lower points. The output streams are called "cuts" (e.g. "side cut", "top cut") and those ones that are not (yet) processed further are called "straight-run" (e.g. "straight-run naphtha"). - The main distilling column in Lekki is the biggest in the world (as of 2022) and with 112.5m height even bigger than the rocket Saturn V or the Eko Tower Black Pearl in Lagos. | 1 | UOP |
| RFCC (Residue Fluid Catalytic Cracking)[16][17][18]
Input: residue fluid from CDU and from Unicracking, air, catalyst, Output: combustible gases, flue gas, catalyst (to be re-used) Purpose: breaking molecules longer than 70 carbon atoms into shorter ones |
This cracking process converts residue fluid coming from the CDU into lighter components (see image below). The residue fluid consists of molecules with many carbon atoms (more than 70) and complex ring and branch structures. It is black, viscous and cannot be evaporated even at high temperatures - making it unusable as a fuel without having been cracked. The residue fluid is brought into contact with a catalyst at high temperature. The catalyst in this procedure is an acidic matrix such as crystalline aluminosilicate zeolite.[19] The cracking takes place, turning the fluid into gas of short-molecule compounds. After this the catalyst is recovered and regenerated. The hydrocarbons are fed to a column that separates them according to their boiling point (and thus molecular length, similar to the CDU).[20] - Because of the geometry of their molecules, cracking of alkanes always means (1.) resulting excess of carbon atoms e.g. in cokers or (2.) necessary addition of hydrogen e.g. in hydrocracking or (3.) resulting unwanted double covalent bonds in hydrocarbon compounds ("alkenes"). Since in RFCC no hydrogen is added, the outgoing compounds belong mostly to the alkenes/olefins (ethene/ethylene, propene/propylene, etc.). They can be hydrotreated into alkanes (for petrol), alkylated or polymerized into polyethylene/polypropylene. The RFCC regenerator in Lekki has been the heaviest item on an African road, before it was installed. It also is the heaviest single piece made of metal in the world. - The RFCC is one of the most safety-critical areas of a refinery. In 2015, the regenerator of an Exxon refinery in Torrance, California, exploded due to lack of maintenance (the expander had worn out, a heat exchanger from a different unit was leaking combustible gases into the system and a catalyst slide valve worked insufficiently after too many years of usage) and incorrect actions after a malfunction occurred (decreasing the steam flow, installing a distance ring and "variance" from standard procedure).[21] In 2018, the Superior refinery in Wisconsin exploded, also due to a worn out RFCC catalyst slide valve.[22] | 1 | UOP |
| "Unicracking" (Hydrocracking) in mild hydrocracking mode[23]
Input: heavy oil from CDU Output: C4...C12 alkanes, fractionated into light and heavy naphtha, jet fuel, Diesel, residue fluid Purpose: breaking molecules longer than 25 carbon atoms into shorter ones |
Hydrocracking[18][24][25][26] "breaks" heavier components (molecules with more than 25 carbon atoms) in the presence of hydrogen into medium-sized molecules (4 - 12 carbon atoms per molecule).[27][28] Mild hydrocracking (MHC) is cracking with less pressure but by using a catalyst in the presence of hydrogen (the pressure is 60 to 110 bar in comparison to conventional hydrocracking at 200 bar). As catalyst Zeolite is being used. Unlike hydrotreating, where hydrogen is used to break bonds between carbon and sulphur or carbon and nitrogen, mild hydrocracking uses hydrogen to break bonds between carbon atoms.[29][30] - Safety rules must be observed here. At the Valero refinery in Delaware, two service technicians died in 2005 from nitrogen asphyxiation while trying to retrieve a roll of duct tape from the reactor, only 5 feet (1.5m) below them.[31] Other workers had previously flooded the reactor with nitrogen to remove oxygen, but had not put up a specific warning. | 1 | UOP |
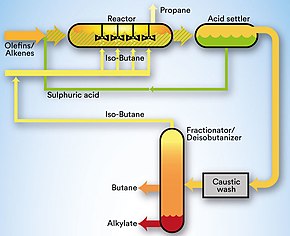
| Alkylation ("Alky")
Input: propylene, butylene (from RFCC), isobutane Output: isoheptane, isooctane (for blending into gasoline), butane, propane Purpose: turning above mentioned components into gasoline blends |
The by-products of the RFCC process are short-chain alkenes (propylene, butylene) and isoparaffins (isobutane), which are not desirable. Alkylation is used to convert isobutane and low molecular weight alkenes (mainly propylene and butylene) into alkylate, mainly isoheptane and isooctane. Alkylate is a high quality gasoline blend because it has good anti-knock properties and burns clean. The process takes place in the presence of an acid - in the Dangote refinery this is sulphuric acid (H2SO4). The plant is called a sulphuric acid alkylation unit (SAAU).[32] The main technology for SAAU is the STRATCO process licensed by DuPont. In the last ten years, more than 85% of the SAAU capacity added worldwide has been produced using STRATCO-DuPont technology. A SAAU can be divided into five major sections: reaction, refrigeration, effluent treating, fractionation and blowdown. In the reactor, the reacting hydrocarbons are brought into contact with the catalyst sulphuric acid at a temperature of 15.6 °C (60 °F). Then the feedstocks are treated to remove impurities, especially water. The feedstock is cooled in the refrigeration section and the light hydrocarbons are discharged from the plant. Then acid, alkyl sulfates and di-alkyl sulfates are removed from the effluent stream to prevent corrosion and fouling in the downstream process. To maintain the desired strength of spent acid, a small amount of fresh acid is continuously added to the reactor. In the fractionation section, the superfluous isobutane is recovered and the remaining hydrocarbons are separated into the desired products. The spent acid is degassed in an acid blowdown drum and the acid effluent is neutralised with caustic in a scrubber before being flared. The spent acid is stored and discharged at regular intervals. - Here, too, safety rules must be observed. On 22 November 2016, a fire occurred in a refinery in Baton Rouge due to maintenance work on a 30-year-old valve that had to be operated manually. 900kg of isobutane escaped and formed an explosive gas cloud that ignited at a welding machine that was left switched on (20m away). 4 workers sustained serious burns.[33] In Philadelphia, Pennsylvania, on 21 June 2019, propane leaked from a manifold at an alkylation plant and ignited, resulting in three extremely violent explosions. This launched several pieces of equipment, including a 19-tonne container that crashed 700 m from its original location.[34] | 1 | DuPont |
| Naphtha Hydrogen Treatment (NHT, "Hydrotreatment")[35][36]
Input: Naphtha from CDU with sulphur and nitrogen contaminations, hydrogen, Output: light naphtha for Penex, heavy naphtha for CCR, ammonia, hydrogen sulphide Purpose: removing sulphur and nitrogen components from gasoline blends (mainly) |
Naphtha is a mixture of hydrocarbon molecules which resemble petrol in the number of their carbon atoms (5 to 12), but some of which have inclusions of sulphur or nitrogen. In this procedure light or heavy naphtha coming from the CDU reacts with hydrogen in the presence of a catalyst like cobalt-molybdenum (or nickel-molybdenum for low-sulphur crude oil) at relatively high temperatures and moderate pressures. (Reactor conditions for a naphtha hydrotreater unit are around 205-260˚C with a pressure of 25-45 bar.[35]) NHT converts olefins / alkenes (like hexene), nitrogen, oxygen, metals and sulphur compounds into products, which can be utilized in other processes.[37] Main purpose of the NHT is to remove sulphur and nitrogen (see images in gallery below). In a combustion engine these fuel contaminations turn into NOx and SOx and are harmful to human health and the environment. Sulphur and nitrogen compounds in naphtha can also deactivate the catalyst in reforming procedures like CCR and therefore must be removed prior to catalytic reforming. - NHT consists mainly of a heater, a hydrogen injector, a fixed-bed reactor and a separator column, in which the desulfurized naphtha and gases like hydrogen and hydrogen sulfide are divided (see image in the gallery below). - The last section of NHT can be a "slide stripper". The slide stripper separates ("strips") light naphtha (pentanes, hexanes) from heavy naphtha.[36] The light naphtha goes directly to gasoline blending or is isomerized first. The slide stripper consists mainly of a stripping column and a reflux drum.[37] Like the RFCC, the NHT is a safety-critical area of a refinery. 2010 the NHT heat exchanger of the Tesoro refinery in Anacortes ruptured, causing an explosion and killing 7 workers. The heat exchanger had suffered long term effects of "high temperature hydrogen attack".[38] The maintenance staff had ignored the leaking of the heat exchanger before the explosion, considering it to be "normal".[39] | 1 | UOP |
| Naphtha splitter column | It splits hydrotreated naphtha into light and heavy naphtha.[40] While the Dangote refinery lists this column as a separate unit, it probably is identical to the "slide stripper" mentioned in section "Naphtha hydrogen treatment" further up in this table. | ||
| Diesel Hydrotreatment (DHDT)
Input: C9...C24, hydrogen, Output: C9...C24, hydrogen sulphide Purpose: removing sulphur and nitrogen components from Diesel |
Diesel Hydrotreatment (DHDT) removes impurities like sulphur and nitrogen from diesel oil in the presence of a catalyst and hydrogen converting it into diesel fuel.[41] Diesel fuel has 9 to 24 carbon atoms per molecule (C9 ... C24), while petrol has 4 to 12 carbon atoms per molecule (C4...C12).[37] Diesel oil also has a higher sulphur content than naphtha. The process of hydrotreating diesel oils is similar as but much more complex than NHT, primarily due to the addition of the regenerative amine system, which recovers excess hydrogen gas and removes hydrogen sulfide via diethanolamine (DEA).[36] | ||
| Continuous Catalyst Regeneration (CCR) Platforming[42][43][44]
Input: heavy naphtha from NHT, hydrogen, Output: combustible gas, BTX (high octane gasoline components), hydrogen surplus Purpose: improving the RON of gasoline |
In high compression engines, compounds such as linear pentane or hexane tend to ignite before the spark plug delivers the spark. This is called "engine knocking" and is undesirable. For each component there is a measure that indicates how "knock-proof" it is: the RON or octane rating. Since naphtha has an octane rating around 90, but modern engines require RON 95 or 98, some of the naphtha must be converted into components that have a RON of over 98 and blended into the petrol. These components are the aromatic compounds: molecules with ring structures and with double covalent bonds. They have a RON between 99 (Benzene), 120 (Xylene) and 120...146 (Toluene).[45] The conversion of naphtha into these aromatic compounds takes place in the CCR (see images in the gallery below). This process converts linear molecules with at least 7 carbon atoms into ring-shaped (aromatic) compounds like BTX (benzene, toluene, xylene) by withdrawing hydrogen from them. Hydrogen is an important by-product of CCR and is used in other procedures on this list. - At first, hydrogen is added to depentanized or dehexanized naphtha under 4 to 45 atm and 495°C to 525°C. The process is highly endothermic, which requires constant re-heating between several reactor chambers. The incoming naphtha must also be free of sulphur since it damages the catalyst (this is called "nickel catalyst poisoning"). After the chemical reaction the stream goes into a separator which extracts excess hydrogen. After this a stabilizing column divides the stream into lighter molecule components (methane, ethane, propane) and the high octane "reformate", similar to a distilling column.[46] Besides Dangote's CCR, the alternative, SR platforming ("semi-regenerative", "fixed-bed"), should be mentioned. However, CCR is considered to be more technically advanced (e.g. the catalyst does not wear out as quickly).[43] | 1 | UOP |
| Penex isomerization processes[47]
Input: light naphtha, Output: gasoline blends with a RON of 92 Purpose: improving the RON of gasoline |
Similar to the CCR, the Penex isomerization improves the octane rating of fuel. Light naphtha with 5 or 6 carbon atoms per molecule (pentane/C5, hexane/C6) has an octane rating (RON) of 50 ... 60, while gasoline at the pump station has a RON of 95 or 98. For this reason the operators of a refinery transform C5 and C6 into compounds with a higher RON.
This process isomerizes light naphtha (pentane, hexane) into higher-octane, branched molecules. The Penex process uses fixed-bed catalysts with chlorides. In a UOP Penex unit the feedstock passes a deisopentanizer (which removes isopentane), then a reaction chamber with a fixed-bed catalyst, then a deisohexanizer (which removes isohexane) and finally a Molex technology column. The end product has a RON of ca. 92. An isomerization unit is also safety critical. 2005, in Texas City, the distilling column of a isom unit erupted and then ignited, killing 15 employees and injuring 180 people.[48] This happened due to a faulty measuring instrument as well as overworked and underqualified personnel. It is the worst accident the US Chemical Safety Board has investigated. |
1 | UOP |
| LPG Splitter | Propane-Propylene (PP) Splitters transform Liquefied Petroleum Gas (LPG) to the heavier Polymer Grade Propylene (PGP) and the lighter propane by using thermocompression.[49] | ||
| Merox treatment
Input: sulphides (corrosive, foul smell) in jet fuel or combustible gas, Output: di-sulphides (harmless, smell of garlic, onions or not at all) to be fractionated out, hydrogen Purpose: removing corrosive sulphide compounds |
Sulphur compounds in crude oil products, the "mercaptans", cause so-called "fouling": in continuous operation, the sulphur decomposes all metallic objects with which it comes into contact and deposits elsewhere together with soot and rust as plaque on the inside of pipes, e.g. heat exchangers - leading to blockages and leaks. Operators of refineries therefore try to eliminate sulphur from their products, even if legal regulations do not stipulate this in this form - for example for kerosene or LPG gas. - In the Merox process, the highly corrosive sulphides are converted into the harmless di-sulphides - a process also known as "sweetening" because (at least the larger-molecule) di-sulphides do not have the foul ("rotten eggs"/"rotten vegetables") odour of sulphides (the shorter molecule di-sulphides smell like garlic or onions). Each sulphur atom is thereby bonded to another sulphur atom and thus largely ineffective against metals. - This is achieved by adding caustic soda or ammonia. The lye removes a hydrogen atom from the sulphur atom in the sulphide, after which it bonds to a hydrogen-free sulphur atom of another sulphide molecule. Di-sulphide molecules are therefore almost twice as long as sulphide molecules (see image below), have a higher boiling temperature and can thus be easily fractionated out of the product.[50][51] | UOP | |
| SCANfining | SCANfining (Selective Catalytic Naphtha hydrofining) is a hydro-desulfurization process (HDS) which selectively removes sulphur from catalytically cracked naphtha with a minimal octane loss.[52] SCANfining uses a Al2O3 catalyst (RT-225) with a low metals content and high dispersion. This achieves high HDS and olefin saturation selectivity.[53][54] | EMRE (Exxon) | |
| FCCG desulfurization treatment[55] | Flue gas treatment reduces the number of pollutants produced from the combustion of fossil fuels. It can contain pollutants such as particulates, mercury, sulfur dioxide and carbon dioxide.[56] | 1 | EMRE (Exxon) |
| Butamer processes[57][58] | Isobutane is a primary component for motor fuel. The Butamer process is a high-efficiency, cost effective means of isomerizing normal butane (nC4) to isobutane (iC4).[59] | 1 | UOP |
| Sulphur recovery[60] (SRU) | SRU recovers sulfur-containing, poisonous acidic gas. The most commonly used process is the Claus Process, in which the incoming gas burns with oxygen and is refrigerated. This recovers sulfur from the burnt gas.[61] | 2 | Jacobs |
| Hydrogen plant | It usually consists of a Steam Methane Reformer (SMR) and a hydrogen purification system. Preheated natural gas (or Refinery Off Gas) is introduced across a catalyst to produce a 75–80% hydrogen stream, then is puried (by a MEA scrubber or a PSA unit) to produce 99% pure hydrogen.[62] | 2 | Air Liquide |
| Polymerization | Polypropylene and polyethylene are the two most well known commodity plastics produced through the polymerization of propylene/ethylene with catalysts.[63] | 2 | INEOS |
| Gas plant[64]
Input: combustible gas from the CDU or RFCC, Output: LPG gas, propane, butane |
The gas plant extracts the heavier and more valuable gases out of gas coming from the distillation units and other process units. There are two types of gas plant. A saturated gas plant treats gas streams that contain only saturated hydrocarbons (mainly methane, ethane, propane and butane). An unsaturated gas plant treats gas from cracking units (RFCC, hydrocracking, coker) that contains unsaturated hydrocarbons (olefins / alkenes like propene / propylene and butene / butylene).[65]
The gas plant leads the more valuable heavy components back into product blending or to conversion and sends the lighter and less valuable gases to the refinery fuel system where they are burned for heating processes like in the endothermic CCR platforming / reforming. The gas plant consists of a number of process units like:[65]
|
Superlatives
In 2019, the world's largest crude distillation column, weighing 2,350 tonnes, was installed in place at the Dangote refinery by a specialist Dutch company.[68] With a height of 112 metres it is slightly taller than the Saturn V rocket which brought the first man to the moon (110.6m) and 16 metres taller than Big Ben. In the same year, three more records were set when the world's heaviest refinery regenerator was installed[69][70] - having already been the "heaviest item ever to be transported over a public road in Africa" at a stately 3,000 tonnes and also being "the heaviest single piece of steel structure" of the world.[71][72] As a part of the RFCC (Residue Fluid Catalytic Cracker) a regenerator boils heavy crude oil components until their molecules break up and turn into lighter molecular components like gasoline, kerosene etc.[69] This improves the efficiency of turning crude oil into more valuable components.
The de-butanizer of the unsaturated gas plant is 50 metres high, has a diameter of 8 meters and weighs 520 tons.[70] An unsaturated gas plant handles streams from cracking units that contain unsaturated olefins such as butylene and propylene.[65][73] The gas plant consists usually of a de-ethanizer, a sponge absorber, a de-butanizer, a de-propanizer and a de-isobutanizer.[65]
The Penex stabilizer column is 50 metres high, has a diameter of 8 metres and weighs 520 tons.[70] In the Penex process the molecules of light straight run naphtha are "isomerized" (rearranged) into molecules of a higher octane level by using a catalyst like aluminium chloride.[74][75]
Marine facilities
The self sufficient marine facility has the ability for freight optimization. To the marine facilities belong:[76]
- 2 crude SPM (single point mooring, see image below) for unloading ships from Aframax to ULCC (ultra-large crude carrier)
- 3 product SPM for product exports up to Suezmax vessels
- 2 subsea crude pipelines (diameter 48" or 1.22 metres) with interconnection
- 4 subsea pipelines for products and imports (diameter 24" or 0.61 metres)
- ca. 120km subsea pipeline
Targeted performance
With a single crude oil distillation unit, the refinery will be the largest single-train refinery in the world.[1]
At full production, the facility will process about 650,000 barrels of crude oil daily, transported via pipelines from oil fields in the Niger Delta, where natural gas will also be sourced to supply the fertilizer factory and be used in electrical generation for the refinery complex.[77][4] This corresponds with 50,000,000 litres (13,000,000 US gal) of Euro-V quality gasoline and 17,000,000 litres (4,500,000 US gal) of diesel daily, as well as aviation fuel and plastic products.[4] With a greater capacity than the total output of Nigeria's existing refining infrastructure, the Dangote Refinery will be able to meet the country's entire domestic fuel demand, as well as export refined products.[77]
Gallery
References
- ^ a b c d e "Billionaire's huge Nigerian oil refinery likely delayed until 2022: sources". Reuters. 10 August 2018. Retrieved 13 October 2018.
- ^ a b "Nigeria's Dangote signs deal to build oil refinery". BBC News Online. 4 September 2013. Retrieved 13 October 2018.
- ^ "Can this massive refinery solve Nigeria's energy crisis?". CNN Money. 6 June 2016. Retrieved 13 October 2018.
- ^ a b c d "A $15 Billion Oil Bet Is Tough Challenge for Richest African". Bloomberg Businessweek. 13 July 2018. Retrieved 13 October 2018.
- ^ a b Diala, Sam; THEWILL (2022-07-21). "Dangote Borrows N187.6bn for Refinery Completion". Retrieved 2022-08-06.
- ^ "Port Harcourt refinery to start operation next year The Nation Newspaper". 2022-04-15. Retrieved 2022-08-06.
- ^ Sadiq, Lami (2021-02-24). "Nigeria: Kaduna Refinery to Resume Operations Q1 2023". allAfrica.com. Retrieved 2022-08-06.
- ^ Maples, Robert E. (2000). Petroleum Refinery Process Economics (2nd ed.). Tulsa, Oklahoma: Pennwell Pub. ISBN 9780878147793.
- ^ "Honeywell awarded equipment contract for Dangote's largest single-train refinery". www.constructionboxscore.com. Retrieved 2022-06-14.
- ^ Treese, Steven A; Pujadó, Peter R; Jones, David S. J, eds. (2015). Handbook of Petroleum Processing. doi:10.1007/978-3-319-14529-7. ISBN 978-3-319-14528-0.
- ^ Petroleum refining processes explained simply, retrieved 2022-06-15
- ^ Crude Distillation Unit, retrieved 2022-06-16
- ^ "How to Get the Best From Your Preheat Train an UK Refinery Based Case Study". www.aiche.org. 2013-04-29. Retrieved 2022-06-23.
- ^ Wilson, Ian. "Mitigation of Crude Oil Refinery Heat Exchanger Fouling Through Retrofits Based on Thermo-Hydraulic Fouling Models". Researchgate. Retrieved 23 June 2022.
- ^ Axens. "CDU (Crude Distillation Unit)". axens.net. Retrieved 2022-06-18.
- ^ Fluid Catalytic Cracking Unit Overview FCCU, retrieved 2022-06-15
- ^ Fluid Catalytic Cracking, retrieved 2022-06-15
- ^ a b C.2.2 - Compare catalytic cracking, thermal cracking and steam cracking, retrieved 2022-06-15
- ^ "Optimisation of product yield and coke formation in a RFCC unit". www.digitalrefining.com. Retrieved 2022-06-30.
- ^ "Optimisation of product yield and coke formation in a RFCC unit". www.digitalrefining.com. Retrieved 2022-06-18.
- ^ Animation of 2015 Explosion at ExxonMobil Refinery in Torrance, CA, retrieved 2022-07-02
- ^ Animation of April 26, 2018, Explosion and Fire at the Husky Energy Refinery in Superior, Wisconsin, retrieved 2022-07-02
- ^ Clarke, Ian. "UOP Hydrocracking Technology - Upgrading Fuel Oil to Euro V Fuels" (PDF). VCM Study. Retrieved 2022-06-16.
- ^ "What is Hydrocracking? - Definition from Corrosionpedia". Corrosionpedia. Retrieved 2022-06-15.
- ^ "Chinese Refiner to Use Honeywell UOP Unicracking". uop.honeywell.com. Retrieved 2022-06-15.
- ^ Hydrocracking, retrieved 2022-06-15
- ^ Bhatia, Subhash (1989-12-21). Zeolite Catalysts: Principles and Applications. CRC Press. ISBN 978-0-8493-5628-5.
- ^ Jones, David S. J.; Pujadó, Peter R.; Pujadó, Peter P. (2006-01-11). Handbook of Petroleum Processing. Springer Science & Business Media. ISBN 978-1-4020-2819-9.
- ^ TOPSOE. "Hydrocracking | Mild hydrocracking". www.topsoe.com. Retrieved 2022-06-19.
- ^ "IsoTherming® Technology for Mild Hydrocracking". Elessent Clean Technologies. Retrieved 2022-06-19.
- ^ CSB Safety Video: Hazards of Nitrogen Asphyxiation, retrieved 2022-07-11
- ^ "Alkylation unit". www.mckinseyenergyinsights.com. Retrieved 2022-07-27.
- ^ Animation of Fire at ExxonMobil's Baton Rouge Refinery, retrieved 2022-07-12
- ^ Preliminary Animation of Philadelphia Energy Solutions Refinery Fire and Explosions, retrieved 2022-07-18
- ^ a b "Naphtha hydrotreater - oil refinery - Naphtha hydrotreater". www.prelectronics.com. Retrieved 2022-06-16.
- ^ a b c "Naphtha Hydrotreating Unit". EnggCyclopedia. 2012-02-11. Retrieved 2022-06-16.
- ^ a b c "An Overview of Hydrotreating". www.aiche.org. 2021-09-23. Retrieved 2022-06-18.
- ^ Animation of Explosion at Tesoro's Anacortes Refinery, retrieved 2022-07-02
- ^ Animation of Explosion at Tesoro's Anacortes Refinery, retrieved 2022-07-02
- ^ "544 tons naphtha splitter successfully relocated". www.hydrocarbonprocessing.com. Retrieved 2022-06-18.
- ^ "Hydrocracker Unit (HCU) | Diesel Hydrotreating (DHT)". yesyen.com. Retrieved 2022-06-19.
- ^ PFD of a catalytic reforming Unit, retrieved 2022-06-23
- ^ a b UOP Platforming Process, retrieved 2022-06-23
- ^ "Continuous Catalytic Reforming (CCR)". www.chromalox.com. Retrieved 2022-06-15.
- ^ Kanellopoulos, Nick (2015). Small-Scale Gas to Liquid Fuel Synthesis. Boca Raton, FL: CRC Press. p. 431. ISBN 1466599391, 9781466599390.
{{cite book}}: Check|isbn=value: invalid character (help) - ^ "Fifty years of CCR platforming". www.digitalrefining.com. Retrieved 2022-06-18.
- ^ "Isomerization Process". Hassan ElBanhawi. Retrieved 2022-06-15.
- ^ Updated BP Texas City Animation on the 15th Anniversary of the Explosion, retrieved 2022-10-01
- ^ "Getting the most from your propane-propylene (PP) splitter unit | Aggreko". www.aggreko.com. Retrieved 2022-06-18.
- ^ GmbH, KROHNE Messtechnik. "Merox treatment in the oil & gas industry". krohne.com. Retrieved 2022-08-08.
- ^ "Merox". www.mckinseyenergyinsights.com. Retrieved 2022-08-08.
- ^ "ExxonMobil's SCANfining Technology Selected for Three Low Sulfur Gasoline Projects for Sunoco, Inc. | Exxon Mobil Corporation". ir.exxonmobil.com. Retrieved 2022-06-24.
- ^ "Detailed Kinetic Model for the Hydro ... - ACS Publications - PDF Free Download". datapdf.com. Retrieved 2022-06-24.
- ^ Hsu, Chang Samuel; Robinson, Paul R. (2007-01-10). Practical Advances in Petroleum Processing. Springer Science & Business Media. ISBN 978-0-387-25789-1.
- ^ "Kashima Refinery - A Barrel Full". abarrelfull.wikidot.com. Retrieved 2022-06-16.
- ^ "Flue Gas Treatment Systems Market Size Share Growth Opportunities and Forecast 2022". DataMIntelligence. Retrieved 2022-06-18.
- ^ "Butamer process". TheFreeDictionary.com. Retrieved 2022-06-15.
- ^ TechStar. "Refining - Butamer Process". info.techstar.com. Retrieved 2022-06-15.
- ^ "UOP BUTAMER". pdfcoffee.com. Retrieved 2022-06-18.
- ^ admin (2022-01-29). "Sulfur Recovery Unit". OilGasPedia. Retrieved 2022-06-16.
- ^ "Sulfur Recovery Unit (SRU) In Refinery, Claus Process Plant Design Engineering". Peiyang Chemical Equipment Co., Ltd. Retrieved 2022-06-18.
- ^ Emerson. "Refining Process Solution Guide" (PDF). www.emerson.com. Retrieved 2022-06-18.
- ^ "Polypropylene | INEOS Polymers". www.ineos.com. Retrieved 2022-06-18.
- ^ www.setlab.com http://www.setlab.com/resources/refining/gas-plants/#1498508139597-fcd50a18-144e. Retrieved 2022-06-15.
{{cite web}}: Missing or empty|title=(help) - ^ a b c d e "Gas plant". www.mckinseyenergyinsights.com. Retrieved 2022-06-14.
- ^ "Fat oil". www.mckinseyenergyinsights.com. Retrieved 2022-06-26.
- ^ www.setlab.com http://www.setlab.com/resources/refining/gas-plants/#1498508139597-fcd50a18-144e. Retrieved 2022-06-18.
{{cite web}}: Missing or empty|title=(help) - ^ Monumental Milestone at Dangote, retrieved 2022-06-13
- ^ a b Odeleye, Femi. "Dangote Refinery's RFCC Unit Gets Installation Of World's Heaviest Regenerator". www.plat4om.com. Retrieved 2022-06-13.
- ^ a b c Dangote Refinery - A Game changer!, retrieved 2022-06-14
- ^ Mammoet. "Mammoet sets a new record in Nigeria". www.mammoet.com. Retrieved 2022-06-13.
- ^ Creating a solid foundation for economic development: Dangote Refinery, retrieved 2022-06-13
- ^ Siddharth, Kumar; Pathak, Amey; Pani, Ajaya Kumar (2019-03-01). "Real-time quality monitoring in debutanizer column with regression tree and ANFIS". Journal of Industrial Engineering International. 15 (1): 41–51. Bibcode:2019JIEI...15...41S. doi:10.1007/s40092-018-0276-4. ISSN 2251-712X. S2CID 52269042.
- ^ Rana Muhammad, Farooq. "Isomerization of Naptha By Penex Process". Retrieved 2022-06-14.
- ^ Naqvi, Salman Raza. "New trends in improving gasoline quality and octane through naphtha isomerization: a short review". Retrieved 2022-06-14.
- ^ Dangote Refinery, retrieved 2022-06-16
- ^ a b "In Nigeria, Plans for the World's Largest Refinery". The New York Times. 9 October 2018. Retrieved 13 October 2018.
- CS1 errors: ISBN
- CS1 errors: missing title
- CS1 errors: bare URL
- Articles with short description
- Short description with empty Wikidata description
- All articles with unsourced statements
- Articles with unsourced statements from August 2022
- Nigeria articles missing geocoordinate data
- All articles needing coordinates
- Articles missing coordinates without coordinates on Wikidata
- Oil refineries in Nigeria
- Proposed energy infrastructure in Africa
- Buildings and structures in Lagos State
- Economy of Lagos State
- Lekki
- Buildings and structures under construction in Nigeria