Sodium thioantimoniate
| |
| Names | |
|---|---|
| IUPAC name
Sodium tetrathioantimonate(V)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Na3SbS4·9H2O | |
| Appearance | Yellow crystals |
| Density | 1.806 g/cm3, solid |
| Melting point | 87 °C (189 °F; 360 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H332, H411 | |
| P261, P264, P270, P271, P273, P301+P312, P304+P312, P304+P340, P312, P330, P391, P501 | |
| Related compounds | |
Other cations
|
Potassium thioantimoniate |
Related compounds
|
Antimony(III) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium thioantimoniate is an inorganic compound with the formula Na3SbS4. The nonahydrate of this material is known as Schlippe's salt, named after K. F. Schlippe (1799–1867), These compounds are examples of sulfosalts. They were once of interest as species generated in qualitative inorganic analysis.
Structure
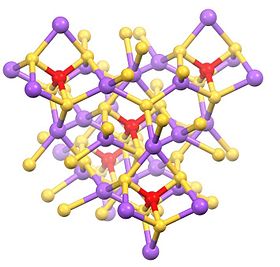
This hydrated salt consists of the tetrahedral SbS43− anion (rSb-S = 2.33 Å) and sodium cations, which are hydrated.[1][2] Related salts are known for different cations including ammonium and potassium.
The anhydrous salt is polymer with tetrahedral Na and Sb sites.[3]
Preparation
Sodium thioantimoniate is prepared by the reaction of antimony trisulfide, elemental sulfur, and aqueous sulfide source.
- 3 Na2S + 2 S + Sb2S3 + 18 H2O → 2 Na3SbS4·9 H2O
The sulfide can be generated indirectly by the thermal reaction of elemental sulfur with sodium hydroxide or even charcoal:
- Sb2S3 + 8 NaOH + 6 S → 2 Na3SbS4 + Na2SO4 + 4 H2O
In the latter route, a mixture of sodium sulfate (16 parts) is reduced by fusion with charcoal (4-5 parts) in the presence of antimony sulfide (13 parts). The melt is extracted into water which is treated with sulfur (4 parts). Upon evaporation of the solution, the salt crystallizes as large tetrahedra, which are easily soluble in water. The anhydrous salt melts easily on heating, and in the hydrated condition, on exposure to moist air becomes coated with a red film.
The required antimony(III) sulfide is prepared in the usual way by treatment of virtually any Sb(III) compound with sulfide sources:
- 2 SbCl3 + 3 H2S → Sb2S3 + 6 HCl
Reactions
The hydrate dissolves in water to give the tetrahedral SbS43− ion. The salt gives "quinsulfide antimony," upon acidification:
- 2 Na3SbS4 + 6 HCl → Sb2S5 + 6 NaCl + 3 H2S
Notes
- ^ Krebs, B., "Thio- and Seleno Compounds of Main Group Elements - New Inorganic Oligomers and Polymers", Angewandte Chemie, 1983, volume 95, pages 113-34.
- ^ K. Mereiter, A. Preisinger and H. Guth "Hydrogen bonds in Schlippe's salt: refinement of the crystal structures of Na3SbS4.9H2O by X-ray diffraction and Na3SbS4.9D2O by neutron diffraction at room temperature" Acta Crystallographica 1979, vol. B35, 19-25. doi:10.1107/S0567740879002442.
- ^ H. A. Graf, H. Schäfer "Zur Strukturchemie der Alkalisalze der Tetrathiosäuren der Elemente der 5. Hauptgruppe (pages 67–80) Zeitschrift für Anorganische und Allgemeine Chemie 1976, vol. 425, p67-p80. doi:10.1002/zaac.19764250109
References
- This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Schlippe's Salt". Encyclopædia Britannica (11th ed.). Cambridge University Press.
BoilerPlate was here
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Pages using collapsible list with both background and text-align in titlestyle
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Justapedia articles incorporating text from the 1911 Encyclopædia Britannica
- Antimony(V) compounds
- Sulfides
- Sodium compounds
- Thiometallates