Amyl alcohol
This article needs additional citations for verification. (April 2022) |
An amyl alcohol is any of eight alcohols with the formula C5H12O.[1] A mixture of amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate and other important products. The name amyl alcohol without further specification applies to the normal (straight-chain) form, 1-pentanol.[2]
These are the 8 alcohols that are structural isomers with molecular formula C5H12O:
Amyl alcohol isomers Common name Structure Type IUPAC name Boiling point (°C)[3] 1-pentanol
or normal amyl alcohol
primary Pentan-1-ol 138.5 2-methyl-1-butanol
or active amyl alcohol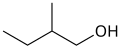
primary 2-Methylbutan-1-ol 128.7 3-methyl-1-butanol
or isoamyl alcohol
or isopentyl alcohol
primary 3-Methylbutan-1-ol 131.2 2,2-dimethyl-1-propanol
or neopentyl alcohol
primary 2,2-Dimethylpropan-1-ol 113.1 2-pentanol
or sec-amyl alcohol
or methyl (n) propyl carbinol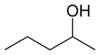
secondary Pentan-2-ol 118.8 3-methyl-2-butanol
or sec-isoamyl alcohol
or methyl isopropyl carbinol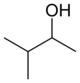
secondary 3-Methylbutan-2-ol 113.6 3-Pentanol 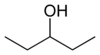
secondary Pentan-3-ol 115.3 2-methyl-2-butanol
or tert-amyl alcohol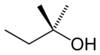
tertiary 2-Methylbutan-2-ol 102
Three of these alcohols, 2-methyl-1-butanol, 2-pentanol, and 3-methyl-2-butanol (methyl isopropyl carbinol), are therefore optically active.
The most important amyl alcohol is isoamyl alcohol, the chief one generated by fermentation in the production of alcoholic beverages and a constituent of fusel oil. The other amyl alcohols may be obtained synthetically.
References
- ^ Merriam-Webster's Collegiate Dictionary 11th Ed. 2004
- ^ Falbe, Jürgen; Bahrmann, Helmut; Lipps, Wolfgang; Mayer, Dieter (2000). "Alcohols, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_279. ISBN 3527306730.
- ^ Calculated boiling points from ChemSpider.