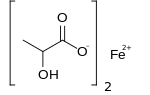
Iron(II) lactate
| |
| Names | |
|---|---|
| IUPAC name
Ferrous 2-hydroxypropanoate
| |
| Other names
Iron dilactate
Iron(II) lactate E585 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10FeO6 | |
| Molar mass | 233.9888 g/mol (anhydrous) 288.03464 g/mol (trihydrate) |
| Appearance | greenish-white powder |
| Melting point | 500 °C (932 °F; 773 K) |
| trihydrate: 2.1 g/100ml (10 °C) 8.5 g/100ml (100 °C) dihydrate: 2% (25 °C)[1] | |
| Solubility | soluble in alkali citrates negligible in alcohol insoluble in ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrous lactate, or iron(II) lactate, is chemical compound with idealized formula Fe(C3H5O3)(H2O)n. No compound has been characterized to establish composition, purity, or structure.
Production
Iron(II) lactate can be produced through several reactions, among which are calcium lactate with iron(II) sulfate according to the following reaction:[citation needed]
Another route yielding iron(II) lactate is to combine lactic acid with calcium carbonate and iron(II) sulfate.
Uses
Iron (II) lactate is used as a reagent in the production of proton-exchange membrane fuel cells (PEMFCs), specifically in the production of cathode catalytic converters used in these cells. It is an acidity regulator and, since it oxidizes on contact with air, it has found use as a color retention agent for foodstuffs such as olives. It is also used to fortify foods with iron, as a remedy for anemia due to iron deficiency, and as a nutritional supplement in tablet or pill form. As a food additive it is coded under the E number E585.[citation needed]
References
- ^ Iron(II) lactate dihydrate MSDS Archived 2014-05-03 at the Wayback Machine at Jost Chemical
- Webarchive template wayback links
- Articles without EBI source
- Articles without KEGG source
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- All articles with unsourced statements
- Articles with unsourced statements from December 2021
- Food additives
- Iron(II) compounds
- E-number additives
- Lactates
- All stub articles
- Organic compound stubs
