Dequalinium
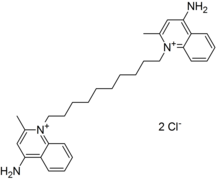
| |
| Names | |
|---|---|
| IUPAC name
1,1'-decane-1,10-diylbis(4-amino-2-methylquinolinium)
decyl]-2-methyl-4-quinolin-1-iumamine dichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| MeSH | Dequalinium |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H40N4 | |
| Molar mass | 456.678 g·mol−1 |
| Pharmacology | |
| D08AH01 (WHO) G01AC05 (WHO), R02AA02 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dequalinium is a quaternary ammonium cation and bolaamphiphile commonly available as the dichloride salt. The bromide, iodide, acetate, and undecenoate salts are known as well. Dequalinium chloride is the active ingredient of several medications:
Dequadin an antiseptic and disinfectant. It is a topical bacteriostat. It is used in wound dressings and mouth infections and may also have antifungal action. It may cause skin ulceration.
Fluomizin, vaginal tablets containing 10 mg dequalinium chloride, are used for treating vaginal bacterial conditions (i.e. Bacterial Vaginosis and aerobic vaginitis).
The dequalinium dication is symmetrical, containing two quaternary quinolinium units linked by an N-decylene chain.
Applications
Dequalinium salts may be used to treat malaria.[1][2] As dequalinium chloride, it can be used in lozenges to cure tonsillitis, but this is not effective in cases of streptococci infections.[3]
References
- ^ Tischer, Maximilian; Pradel, Gabriele; Ohlsen, Knut; Holzgrabe, Ulrike (2012-01-02). "Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions?". ChemMedChem. 7 (1): 22–31. doi:10.1002/cmdc.201100404. ISSN 1860-7187. PMID 22113995. S2CID 26326417.
- ^ "US Patent 4946849". United States Patents and Trademarks Office (USPTO). 10 October 1989. Retrieved 2008-03-15.
- ^ Krämer W. (1977). "[Treatment of tonsilitis with dequalinium chloride]". Fortschr. Med. (in German). 95 (16): 1108–10. PMID 856702.
Further reading
- Gamboa-Vujicic, Gisela; Emma, Dennis A.; Liao, Shu Y.; Fuchtner, Carlos; Manetta, Alberto (1993). "Toxicity of the mitochondrial poison dequalinium chloride in a murine model system". Journal of Pharmaceutical Sciences. 82 (3): 231–5. doi:10.1002/jps.2600820302. PMID 8450414.
External links
- International Mark - Fluomizin(804560)[permanent dead link]
- Medinova Fluomizin
- Comparative Study of Efficacy of 10 mg Dequalinium Chloride (Fluomizin) in the Local Treatment of Bacterial Vaginosis
- CS1 German-language sources (de)
- Articles without KEGG source
- Articles with changed EBI identifier
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Articles with short description
- All articles with dead external links
- Articles with dead external links from July 2019
- Articles with permanently dead external links
- Quinolines
- Quaternary ammonium compounds
- Antiseptics
- All stub articles
- Genito-urinary system drug stubs