1,6-Dichloro-1,6-dideoxyfructose
(Redirected from 1,6-dichloro-1,6-dideoxyfructose)
This article needs additional citations for verification. (February 2021) |

| |
| |
| Names | |
|---|---|
| IUPAC name
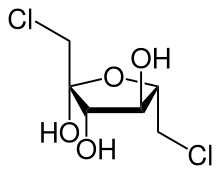
(3S,4S,5S)-1,6-dichloro-3,4,5-trihydroxyhexan-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10Cl2O4 | |
| Molar mass | 217.04 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,6-Dichloro-1,6-dideoxyfructose (dichlorodideoxyfructose) is chlorinated derivative of the sugar fructose. It is one of the two components believed to comprise the disaccharide sucralose, a commercial sugar substitute.
Metabolism
In mammals, 1,6-dichloro-1,6-dideoxyfructose is metabolized in the liver and erythrocytes by a reaction with glutathione that replaces one of the chlorine atoms, forming 6-chlorofructos-1-yl glutathione (or chlorofructosyl glutathione).[1]
References
- ^ Hughes, H M; Powell, G M; Snodin, D J; Daniel, J W; Crawford, A; Sanders, J K; Curtis, C G (15 April 1989). "Glutathione-dependent dechlorination of 1,6-dichloro-1,6-dideoxyfructose". Biochemical Journal. 259 (2): 537–543. doi:10.1042/bj2590537. PMC 1138541. PMID 2719664.
Categories:
- Articles needing additional references from February 2021
- All articles needing additional references
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Articles with short description
- Organochlorides