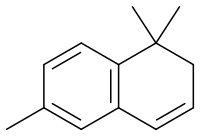
1,1,6-Trimethyl-1,2-dihydronaphthalene
| |
| Names | |
|---|---|
| IUPAC name
1,1,6-Trimethyl-1,2-dihydronaphthalene
| |
| Other names
1,2-Dihydro-1,1,6-trimethylnaphthalene
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TDN |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H16 | |
| Molar mass | 172.271 g·mol−1 |
| Boiling point | 115 °C (239 °F; 388 K) at 18 Torr[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) is an aroma compound present in wine,[1] particularly aged Rieslings.[2][3] Chemically, it is classified as a 13C-norisoprenoid, as it has thirteen carbon atoms, and is derived from an isoprenoid by the loss of methylene groups.[4]
In wines, 1,1,6-trimethyl-1,2-dihydronaphthalene is generally considered to contribute to a desirable aroma in low concentrations, but an undesirable aroma in higher concentrations.[5] The aroma is commonly described as a petrol note or by the French term goût de pétrole.[6]
1,1,6-Trimethyl-1,2-dihydronaphthalene is believed to be a degradation product of β-carotene and lutein.[4] 1,1,6-Trimethyl-1,2-dihydronaphthalene can also by synthesized in the laboratory from either of the ionones, α-ionone or β-ionone.[1]
References
- ^ a b c Dobrydnev, Alexey; Tarasov, Andrii; Müller, Nikolaus; Volovenko, Yulian; Rauhut, Doris; Jung, Rainer (2020). "An optimized method for synthesis and purification of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN)". MethodsX. 7: 56–61. doi:10.1016/j.mex.2019.12.009. PMC 6938800. PMID 31908985.
- ^ Sacks, Gavin L.; Gates, Matthew J.; Ferry, Francois X.; Lavin, Edward H.; Kurtz, Anne J.; Acree, Terry E. (2012). "Sensory Threshold of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and Concentrations in Young Riesling and Non-Riesling Wines". Journal of Agricultural and Food Chemistry. 60 (12): 2998–3004. doi:10.1021/jf205203b. PMID 22397689.
- ^ Dein, Melissa; Kerley, Trenton; Munafo, John P. (2021). "Characterization of Odorants in a 10-Year-Old Riesling Wine". Journal of Agricultural and Food Chemistry. 69 (38): 11372–11381. doi:10.1021/acs.jafc.1c04196. PMID 34547201. S2CID 237596110.
- ^ a b Waterhouse Lab (2016). "TDN, (1,1,6,-trimethyl-1,2-dihydronapthalene)". University of California, Davis.
- ^ Cory Black, Leigh Francis, Prue Henschke, Dimitra Capone, Samantha Anderson, Martin Day, Helen Holt, Wes Pearson, Markus Herderich and Dan Johnson (2012). "Aged Riesling and the development of TDN" (PDF). The Australian Wine Research Institute.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Owen Bird (2005). Rheingold - The German Wine Renaissance. Arima Publishing. pp. 90–97. ISBN 978-1-84549-079-9.
- CS1 maint: multiple names: authors list
- Articles without InChI source
- Articles without KEGG source
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Flavors
- Terpenes and terpenoids
- Oenology